NCERT Solutions Class 11 Chemistry Chapter 4
NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular State - PDF Download
Get set for your success in upcoming CBSE Exams with Vidyakul’s Ncert Solution for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular State. Free PDF downloads are available for the students to clarify their doubts regarding Chapter 3 CHEMICAL BONDING AND MOLECULAR STATE.
The major topics covered in Class 11 NCERT Chapter 4 Chemical Bonding and Molecular State is as follows:
- Kossel-
lewis approach to chemical bonding - Octet rule
- Covalent bond
- Lewis representation of simple molecules (the
lewis structures) - Formal
charge - Limitations of the octet rule
- Ionic or electrovalent bond
- Lattice enthalpy
- Bond parameters
- Bond length
- Bond angle
- Bond enthalpy
- Bond order
- Resonance structures
- Polarity of bonds
- The valence shell electron pair repulsion (
vsepr ) theory - Valence bond theory
- Orbital overlap concept
- Directional properties of bonds
- Overlapping of atomic orbitals
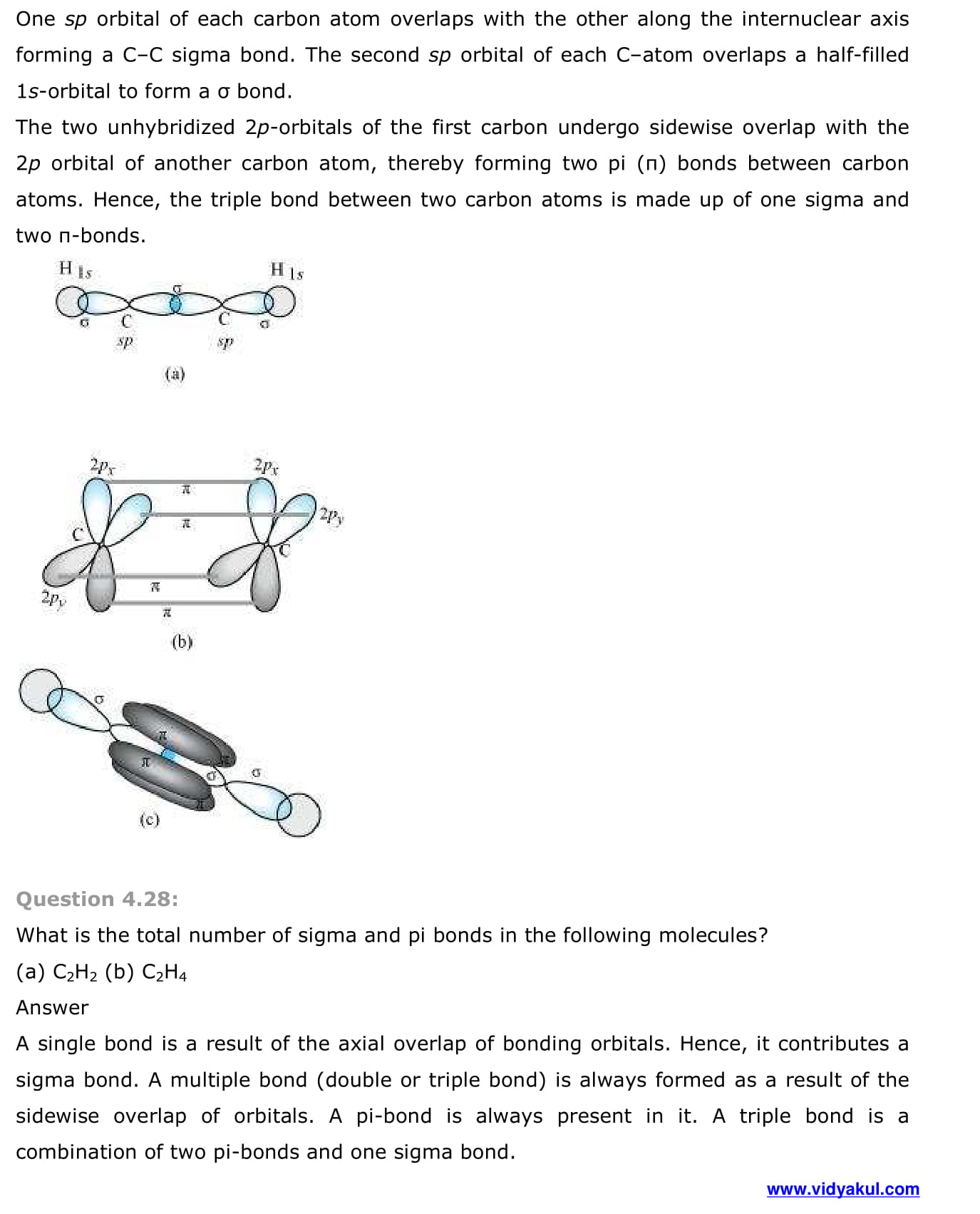
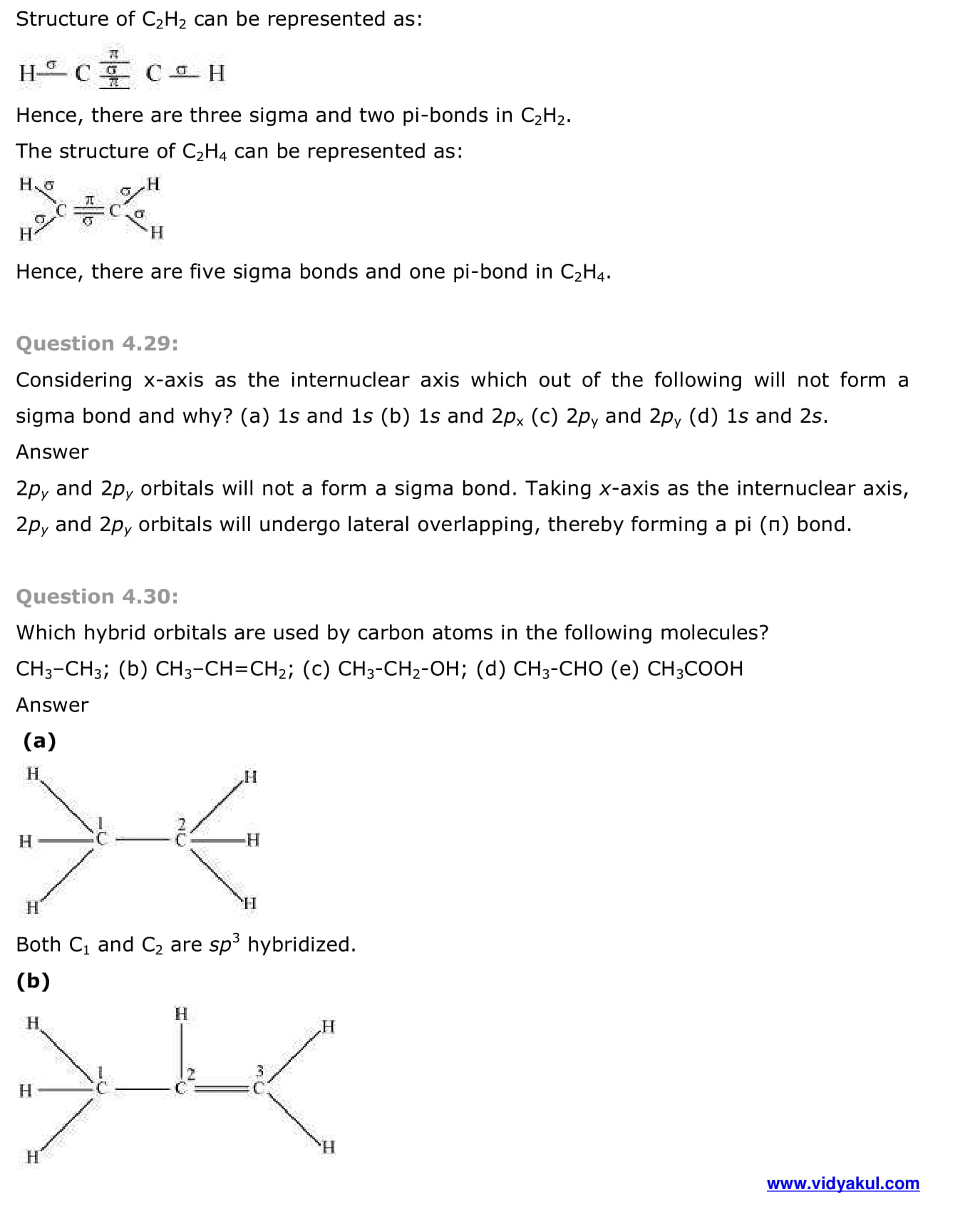
- Types of overlapping and nature of covalent bonds
- Strength of sigma and pi bonds
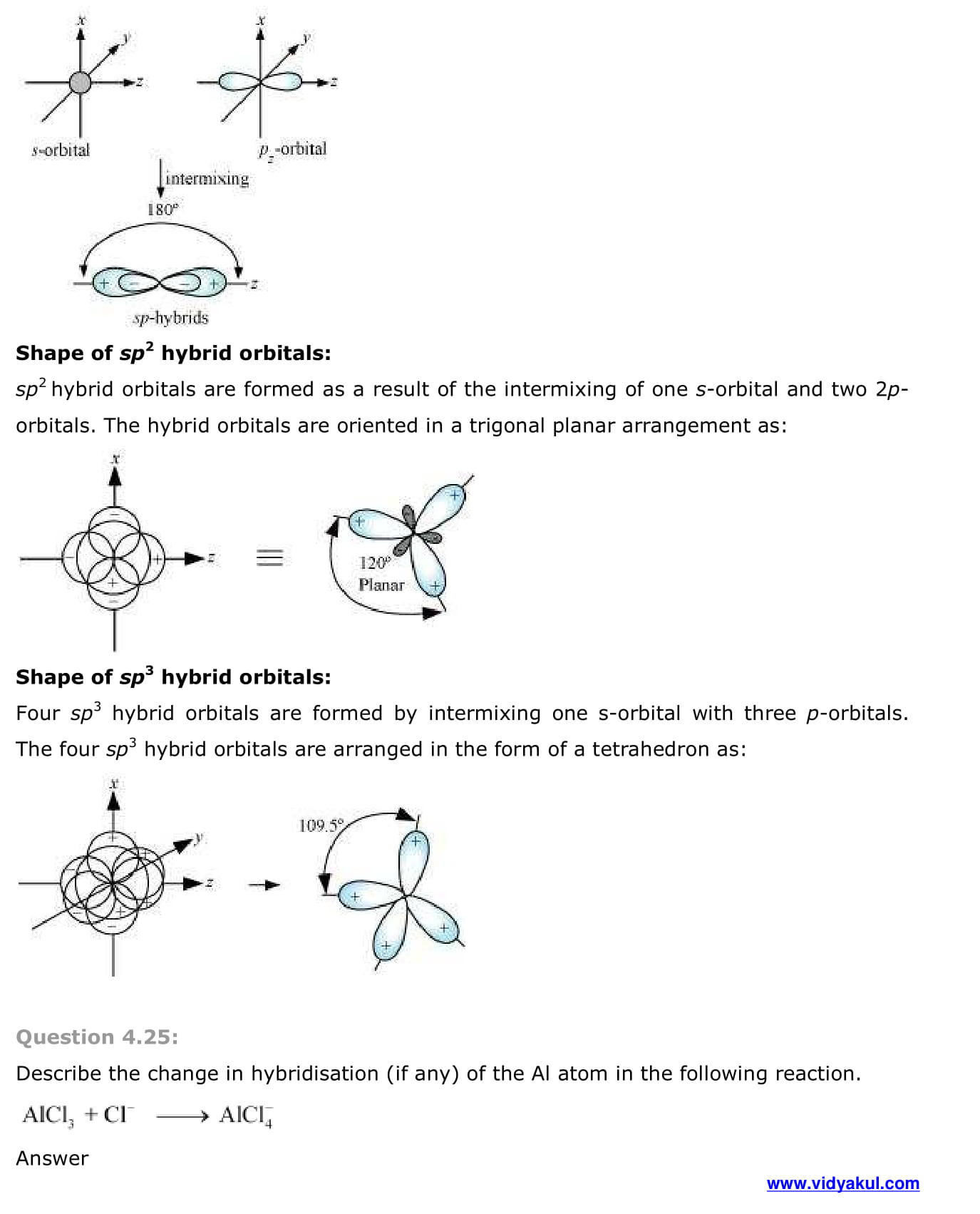
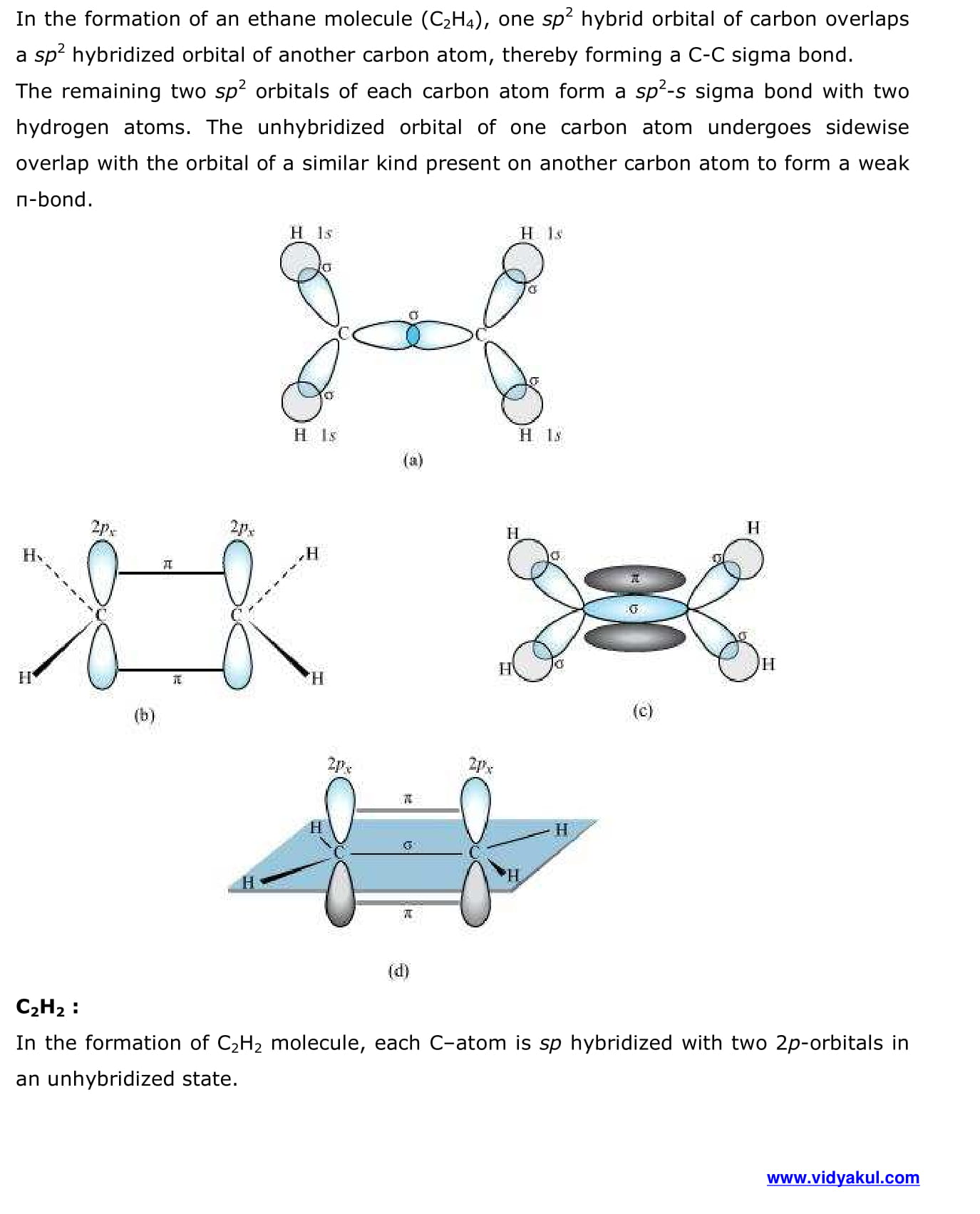
- Hybridisation
- Types of hybridisation
- Other examples of sp3, sp2 and sp hybridisation
- Hybridisation of elements involving d orbitals
- Molecular orbital theory
- Formation of molecular orbitals linear combination of atomic orbitals (
lcao ) - Conditions for the combination of atomic orbitals
- Types of molecular orbitals
- Energy level diagram for molecular orbitals
- Electronic configuration and molecular behaviour
- Bonding in some homonuclear diatomic molecules
Follow the link given below to download the PDF free of cost.
Download this solution for FREE Download this PDF