Chemical Reactions and Equations Class 10 Notes

Chapter 1 Chemical Reactions and Equations
The first chapter of NCERT Class 10 Science is important because the concepts in this chapter relate to other chapters as well. Vidyakul provided NCERT notes for 10th grade to help students better understand concepts and score well on exams. NCERT notes Class 10 Students who are good at Science can easily pass the exam. Learning these notes helps students understand how to solve problems. Students can also visit Vidyakul to practice for free on over 3,240 questions from 103 different test prep books. Read on to learn more about the NCERT notes for Class 10 Chapter 1.
CBSE CLASS 10th SCIENCE CH-1
Points to Remember
We have provided some important points to remember from 10th NCERT Science Chapter 1 for students to refer to for exam preparations:
Changes in which new substances are formed with entirely new properties are called chemical changes/chemical reactions.
Chemical reactions are characterised by some easily observable features like evolution of a gas, formation of precipitate and change in colour, temperature or state.
The reactions in which heat is evolved are known as exothermic reactions while the reactions in which heat is absorbed are known as endothermic reactions.
A balanced chemical equation is an equation which has an equal number of atoms of each element on both reactant and product sides.
A chemical equation can be made more informative by mentioning physical states of the substances involved, heat changes involved in the reaction and conditions under which the reaction takes place.
The reaction in which two or more reactants combine together to form a single product is called a combination reaction. In all combination reactions, heat is evolved i.e., all combination reactions are exothermic.
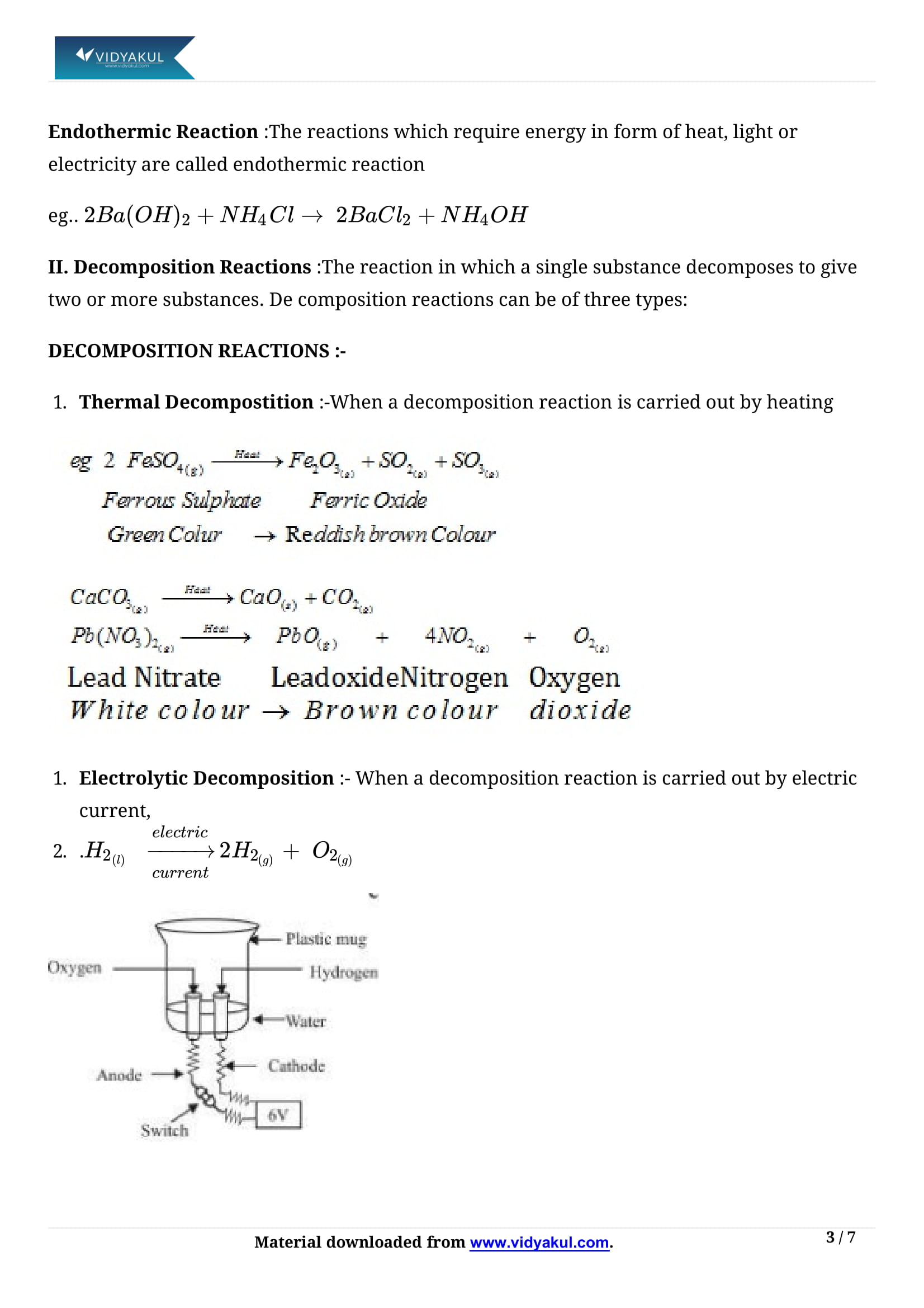
The reaction in which a single reactant breaks down to give two or more simpler products is called a decomposition reaction. For decomposition reactions, energy must be supplied either in the form of heat, light or electricity i.e., decomposition reactions are endothermic.
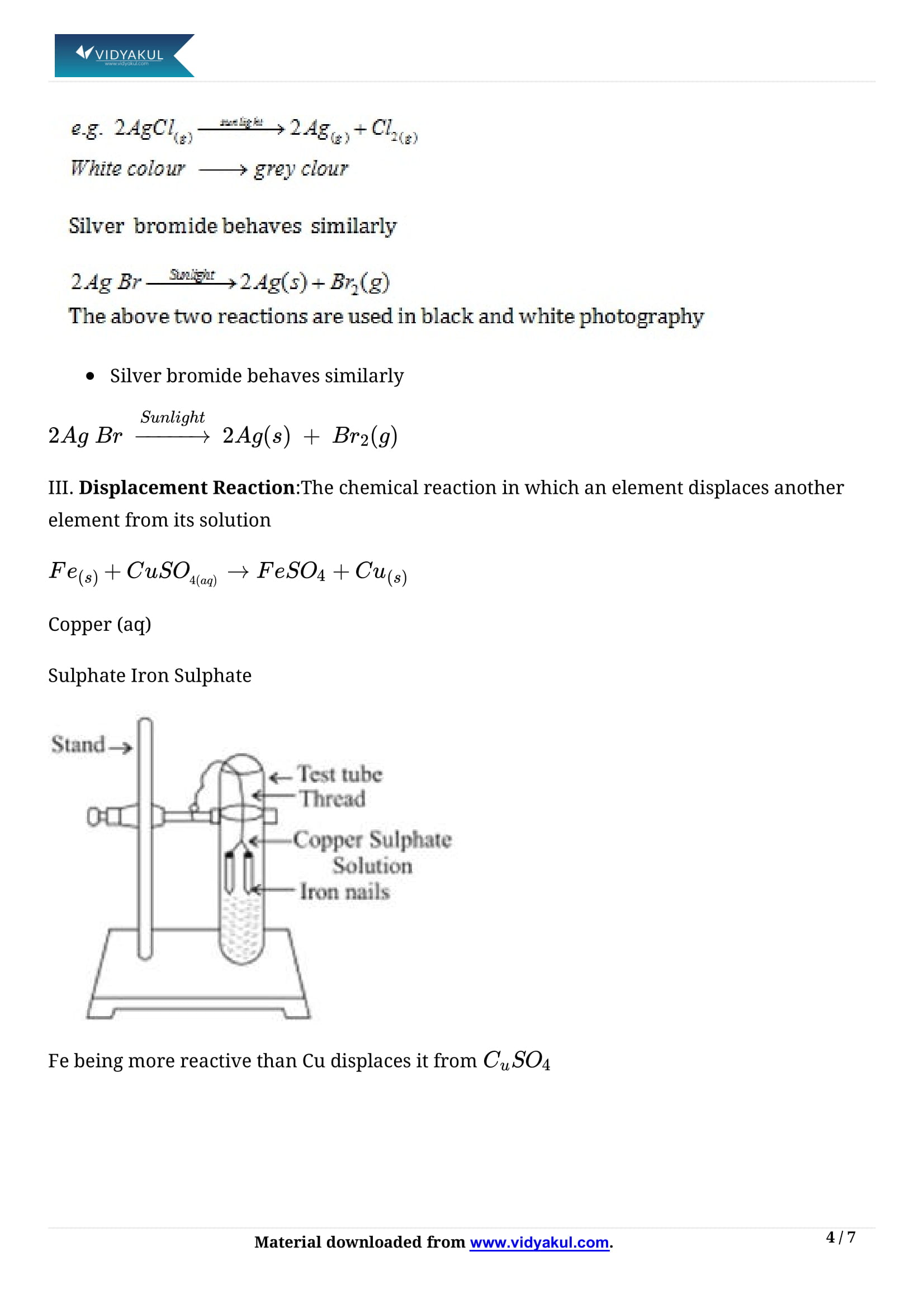
The reaction in which a more reactive element displaces a less reactive element from its compound is called displacement reaction.
The reaction in which two compounds react by an exchange of ions to form two new compounds is called double displacement reaction.
Topics and Sub-topics
In this chapter, students learn about the chemical changes that occur in their surroundings. Learn how substances react with each other to form new substances with new properties and how these changes are expressed in chemical equations. There are many types of chemical reactions. Substances can combine to form new substances or break down to form new substances. Elements can be replaced by other substances, or elements can be replaced to form various compounds. All of these chemical reactions involve the release or absorption of energy. In this chapter, students also learn common examples of such reactions, such as rust and corrosion.
Below, we have provided the list of topics included in this chapter:
Few Important Questions
Carbon reacts with oxygen to give carbon dioxide. This is an example of which type of reaction?
This is an example of combination reaction since two reactants are combining to form a single product.
Identify the type of chemical reaction taking place when silver chloride turns black on exposure to sunlight.
It is a decomposition reaction that occurs in the presence of sunlight and hence it is a photochemical decomposition reaction.
In electrolysis of water (acidified), the name the gases that are evolved at anode and cathode respectively?
In electrolysis of water (acidified), the gases that are evolved at anode and cathode respectively are oxygen and hydrogen. Hydrogen ions gain electrons from cathode and forms hydrogen gas, oxygen ions gives electrons to anode and forms oxygen gas.
Changes in which new substances are formed with entirely new properties are called chemical changes/chemical reactions.
Chemical reactions are characterised by some easily observable features like evolution of a gas, formation of precipitate and change in colour, temperature or state.
The reactions in which heat is evolved are known as exothermic reactions while the reactions in which heat is absorbed are known as endothermic reactions.
A balanced chemical equation is an equation which has an equal number of atoms of each element on both reactant and product sides.
A chemical equation can be made more informative by mentioning physical states of the substances involved, heat changes involved in the reaction and conditions under which the reaction takes place.
The reaction in which two or more reactants combine together to form a single product is called a combination reaction. In all combination reactions, heat is evolved i.e., all combination reactions are exothermic.
The reaction in which a single reactant breaks down to give two or more simpler products is called a decomposition reaction. For decomposition reactions, energy must be supplied either in the form of heat, light or electricity i.e., decomposition reactions are endothermic.
The reaction in which a more reactive element displaces a less reactive element from its compound is called displacement reaction.
The reaction in which two compounds react by an exchange of ions to form two new compounds is called double displacement reaction.
Carbon reacts with oxygen to give carbon dioxide. This is an example of which type of reaction?
Identify the type of chemical reaction taking place when silver chloride turns black on exposure to sunlight.
In electrolysis of water (acidified), the name the gases that are evolved at anode and cathode respectively?
Learn more about it in Chemical Reactions and Equation Class 10 Notes pdf.
Download Latest Class 10 Sample Papers 2019 for Board Exams
- CBSE Class 10 Sample Papers 2019 PDF
- CBSE Class 10 Maths Sample Papers 2019 PDF
- CBSE Class 10 Science Sample Papers 2019 PDF
- CBSE Class 10 English Sample Papers 2019 PDF
- CBSE Class 10 Hindi Sample Paper 2019 PDF
- CBSE Class 10 Social Studies Sample Papers 2019 PDF
Download this solution for FREE Download this PDF
Download Vidyakul App for more notes, pdf and free video lectures.