Periodic Classification of Elements Class 10 Notes

Chapter 5 Periodic Classification of Elements
Periodic classification of elements is the process of classifying elements into different classes. This strategy compares the characteristics of multiple items to group similar items and isolate unrelated items. This helps us understand how different compounds combine to form different elements.
Chapter 5 of NCERT notes for Class 10 Science is devoted to periodic classification of elements. Students coming for exams should pay attention to these topics. As a result, this will help students get more grades. It is also designed according to the latest NCERT program.
CBSE CLASS 10th SCIENCE CH-5
Points to Remember
Given below are some of the most important points about the NCERT notes for Class 10 Science Chapter 5:
The classification of elements is required for a convenient and systematic study of the behaviour and nature of substances.
The modern periodic table is divided into 7 horizontal rows called periods and 18 vertical columns called groups.
s-and p-block elements are called representative elements.
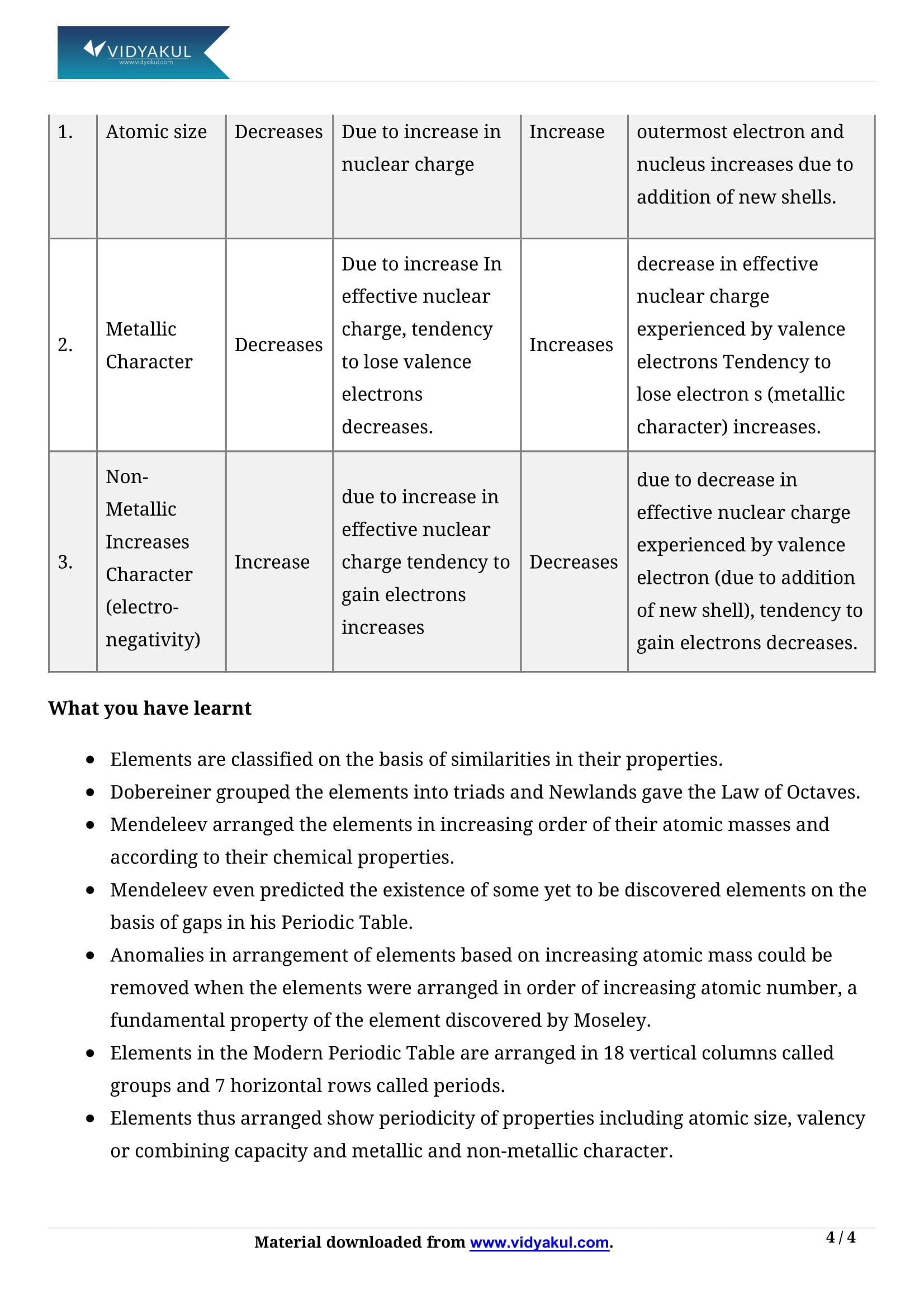
In a period, the number of valence electrons increases on moving from left to right.
In a group, the atomic radii increase with an increasing atomic number from top to bottom.
For more important information, students can refer to Vidyakul.
Topics and Sub-topics
Vidyakul's NCERT Class 10 Science Chapter 5 notes will help students develop the right approach to answer writing. It contains over 500 questions from over 30 books to help students get higher grades. This allows students to practice the questions in this book. All of these materials are provided free of charge by Vidyakul.
Let us look at the topics that students are going to study in this chapter:
Few Important Questions
When was the periodic table framed by Mendeleev?
Mendeleev framed the periodic table in the year 1869.
What is a Halogen?
A Halogen is a chemical element that forms a salt when it reacts with a metal.
What is Electronegativity?
The tendency of an atom (present in a chemical element) to attract shared electrons when forming a chemical bond.
The classification of elements is required for a convenient and systematic study of the behaviour and nature of substances.
The modern periodic table is divided into 7 horizontal rows called periods and 18 vertical columns called groups.
s-and p-block elements are called representative elements.
In a period, the number of valence electrons increases on moving from left to right.
In a group, the atomic radii increase with an increasing atomic number from top to bottom.
When was the periodic table framed by Mendeleev?
What is a Halogen?
What is Electronegativity?
Learn more about in Periodic Classification of Elements Class 10 Notes pdf.
Download Latest Class 10 Sample Papers 2019 for Board Exams
- CBSE Class 10 Sample Papers 2019 PDF
- CBSE Class 10 Maths Sample Papers 2019 PDF
- CBSE Class 10 Science Sample Papers 2019 PDF
- CBSE Class 10 English Sample Papers 2019 PDF
- CBSE Class 10 Hindi Sample Paper 2019 PDF
- CBSE Class 10 Social Studies Sample Papers 2019 PDF
Download this solution for FREE Download this PDF
Download Vidyakul App for more videos, PDF's and Free video lectures.